


- brand:
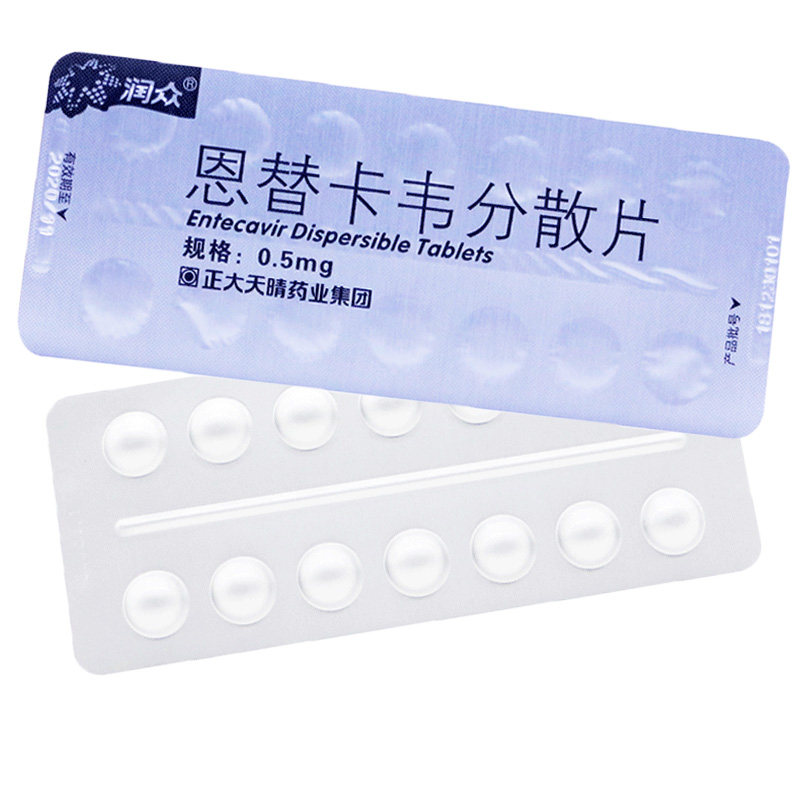
- Runzhong
- Approval number:
- Gyzz h20100019
- Package type:
- 2 boxes 3 boxes 4 boxes 6 boxes standard
- manufacturing enterprise:
- Zhengda Tianqing Pharmaceutical Group Co., Ltd
- Product dosage form:
- tablet
- usage:
- Oral administration
- Usage dose:
- 1 tablet per day
- General Name of Drug:
- Entecavir dispersible tablets
- Drug name:
- Entecavir dispersible tablets
- Term of validity:
- 36 months
- Intended for:
- Adult
- Drug category:
- Chemical medicine
- Drug classification:
- Prescription
- disease:
- hepatitis B
- Symptom:
- hepatitis B
- Drug specification:
- 0.5mg * 28 pieces / box